CDConnectTM
All Your Research Data in One Secure Unified Decentralized Clinical Trial (DCT) Platform
Effortlessly integrate, monitor, and analyze real-time data from wearables and in-home medical devices. Uncover hidden insights to make data-driven conclusions. All in one easy-to-use platform.
Experience the future of clinical trials with our intuitive platform.
Managing clinical trial data in DCTs can be challenging.
- Maintaining patient engagement and adherence to the study protocol can be demanding in a remote setting.
- Ensuring the accuracy and reliability of remotely collected data requires robust data quality monitoring and validation processes.
- Protecting sensitive patient data in a decentralized environment requires strict security measures and compliance with data privacy regulations.
- Ensuring seamless data exchange between different systems and devices can be challenging, especially in a decentralized setting.
- Extracting meaningful insights from clinical trial data can be challenging without advanced data analysis tools to streamline interpretation and decision-making.
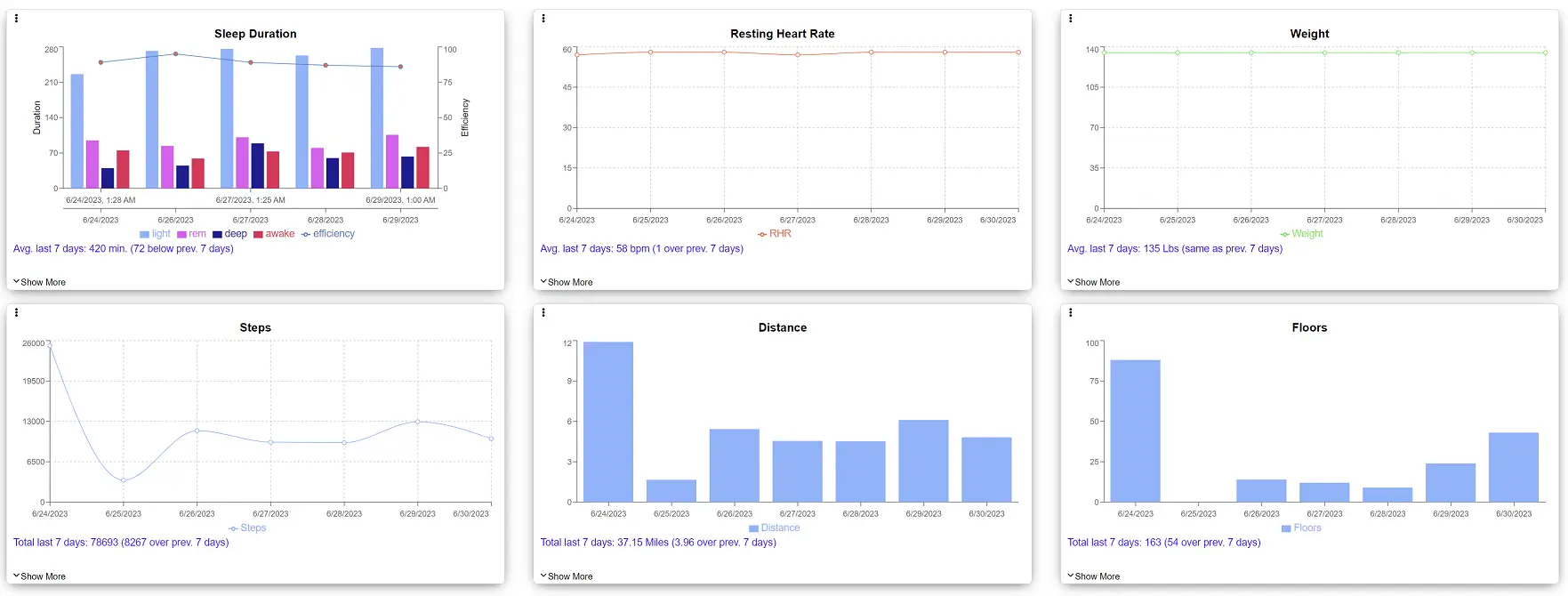
One Powerful Platform, Seamless Data Integration
CDConnect™ is a specialized tool designed for clinical trials that revolutionizes electronic data capture (EDC) by connecting healthcare wearables and in-home medical devices to one secure, efficient, and user-friendly online system. It enables effortless tracking of participant metrics from multiple devices in real-time, monitoring of long-term trends, and comparison of data to identify potential patterns. With the power of continuous data collection and analysis, CDConnect™ enables informed decision-making and improves clinical research outcomes.
CDConnect™: Tailored for Decentralized Clinical Trials
CDConnect is designed to support modern, decentralized clinical trials, enabling seamless remote monitoring and data collection without compromising accuracy or compliance.
How CDConnect™ Optimizes Your Clinical Trial Workflow
- Real-Time Monitoring: Effortlessly track participant metrics remotely, ensuring real-time data anytime, anywhere
- Seamless Device Integration: An easy-to-use interface enables effortless connection of diverse wearables and medical devices, streamlining data collection and management
- Streamlined Data Analysis: Identify trends, patterns, and anomalies to make data-driven decisions that enhance clinical trial efficacy
- Data Visualization: Easily manage and visualize participant data, enabling you to swiftly detect deviations and adapt to changing clinical trial conditions
- Customization: Our platform also offers personalized dashboards, alerts, and reports to meet your clinical trial requirements
- Strict Security Measures: Data is managed with the highest security and regulatory compliance levels to maintain participant confidentiality and trial integrity.
- Global Scalability: Designed for decentralized trials, CDConnect™ supports global operations without needing in-person participant monitoring.
Empower your team with cutting-edge tools that complement traditional EDC systems, seamlessly integrating data from healthcare wearables and in-home medical devices to revolutionize clinical trial research.
CDConnect™ Can Help
Contract Research Organizations (CROs)

Ensure streamlined data integration and timely insights to support sponsor decision-making and reduce cost in multi-site clinical studies
Pharmaceuticals Companies

Download huge datasets collected over weeks or months of a clinical trial in an instant.
Hospitals / Academic Medical Centers

Keep track of participants or patients without the hassle of in-person meetings.
Clinical Trials Companies

Build a database to collect and analyze clinical trial data from participant devices and wearables.
Life Sciences and Biotech Organizations

Deliver data integration solutions for innovative medical devices and global product testing
CDConnect™ DCT Solutions
Clinical Trial Data Management
CDConnect™ streamlines clinical trial data management by automating data collection, ensuring data accuracy, and providing real-time access to information. This leads to increased efficiency, reduced errors, and faster decision-making throughout the clinical trial process.
Electronic Data Capture (EDC)
Our specialized software complements traditional EDC systems and facilitates remote data entry, making it easier for sites to collect data from patients. With enhanced data security measures, CDConnect™ produces detailed reports and visualizations to aid data interpretation.
Clinical Trial Data Analysis
CDConnect™ streamlines clinical trial data analysis by ensuring consistent and comprehensive data collection from multiple devices. By centralizing and organizing data, it provides a solid foundation for users to perform their own analyses. CDConnect™ also offers tools for data exploration and visualization, making it easier to identify trends and generate actionable insights.
How CDConnect™ Works
① Device Integration
Start by adding participants to the study and connecting wearables or in-home medical devices to the CDConnect platform
The interface ensures a smooth onboarding experience for devices, guaranteeing compatibility and smooth data transmission. For instance, devices like smartwatches for heart rate monitoring, blood glucose monitoring devices, or pill dispensers for medication adherence can be synced instantly.


② Data Collection and Monitoring
CDConnect captures participants’ metrics in real time. A secure dashboard provides centralized access to visualize these live updates, so you can easily monitor each participant’s status. In addition, customizable alerts help detect deviations to ensure rapid intervention when needed.
③ Data Analysis and Reporting
Collected data is analyzed using built-in comparison tools to identify patterns, trends, or anomalies. The system supports the rapid generation of trial reports in exportable formats for team collaboration.
Moreover, continuously generated insights help you to make proactive decisions and adjust trial parameters as needed.


④ Collaborative Feedback Loop
We work with you to refine and customize platform features based on specific trial needs. To enhance CDConnect’s usability further, we ask for regular feedback about device integrations or workflow optimizations.
Our Mission Is To…
“…empower researchers and data analysts by simplifying the capture, integration, and analysis of clinical trial data from wearables and in-home medical devices, enabling smarter, faster, and more reliable research outcomes.“
Let’s Shape the Future of Medical Research Together!
We call all doctors, healthcare professionals, clinical trials experts, and investors! We invite you to join our mission to revolutionize the clinical trials landscape.
We call all doctors, healthcare professionals, clinical trials experts, and investors! We invite you to join our mission to revolutionize the clinical trials landscape.
Your valuable ideas and feedback can help shape the future of CDConnect and make it better for medical and research communities.
We’re passionate about fostering a collaborative environment and making a meaningful impact on clinical trial management. We’re eager to hear from you, learn about your experiences, and understand how we can better serve your needs.
Let’s work together to shape the future of medical research!
Ready to transform your clinical research operations?
Handle your clinical trial data more efficiently.
Yes to a faster and more accurate data management system.
Collaborate with us and start optimizing your clinical trial process.
Frequently Asked Questions
Clinical trial data management is a part of clinical trial management that is concerned with collecting, cleaning, and managing data from clinical trials. It involves ensuring the accuracy, completeness, and consistency of data collected throughout the trial. This process is crucial for generating reliable results and making informed decisions about drug development and medical treatments.
Clinical data management emerged as a response to the pharmaceutical industry’s need for rigorous and reproducible research. To ensure data integrity and compliance with regulatory standards, drug companies adopted structured approaches to data collection, analysis, and reporting. This evolution has led to the widespread use of software-based systems that streamline data management, enhance data quality, and speed up clinical trial timelines.
CDConnect™ is ideal for clinical research organizations (CROs), pharmaceutical companies, academic institutions, and medical device manufacturers conducting clinical trials, especially decentralized or hybrid studies.
Yes, CDConnect™ integrates seamlessly with wearable devices and in-home medical devices, allowing for a comprehensive and unified data collection process.
Absolutely. CDConnect™ ensures compliance with evolving regulatory requirements, including GCP, ICH, FDA guidelines, and other regional standards, streamlining audit readiness and regulatory submissions.
Yes, CDConnect™ is committed to safeguarding your clinical trial data.
We take data security seriously, so we partner with a leading cybersecurity firm to conduct regular audits, ensuring the highest level of protection for your study data. Our clinical research data management software has achieved an A+ cybersecurity certification, reflecting our commitment to industry-best practices.
To further safeguard your research data, we leverage Amazon Web Services (AWS) in our cloud-based system and implement robust security measures. Our cloud-based solutions prioritize confidentiality by assigning unique participant IDs, eliminating the need for additional personal information.
CDConnect™ is designed for ease of implementation with intuitive onboarding, dedicated support, and integration capabilities to align seamlessly with existing systems and processes, even in ongoing trials.
Yes, CDConnect™ is specifically designed to support decentralized and hybrid clinical trials. Its capabilities include remote data collection, seamless integration with patient devices, and tools to enhance patient engagement and trial oversight.
Yes, CDConnect™ is scalable and optimized for managing multi-site clinical trials. Its robust features ensure consistent data quality and compliance across all trial locations.
Yes, at CDConnect™, we prioritize the confidentiality and security of your clinical trial data.
To ensure the highest level of security, we partner with a highly reputable third-party cybersecurity firm to conduct rigorous audits of our systems. Our platform has earned an A+ cybersecurity certification, reflecting our commitment to safeguarding your data with industry-leading standards and practices.
We leverage Amazon Web Services (AWS) and implement robust data security measures to safeguard your information. Confidentiality and blinding are maintained by assigning unique participant IDs during registration. No additional personal information is required to start collecting data.
CDConnect™ enhances participant engagement by offering flexible, remote monitoring and easy-to-use tools that simplify data collection processes. The platform empowers participants with virtual trial capabilities, ensuring their involvement without adding unnecessary burdens. This efficient approach not only improves engagement but also supports better compliance with trial protocols.
Yes, CDConnect™ is designed to reduce the operational burden of managing decentralized and hybrid clinical trials. By centralizing trial activities and integrating real-time data monitoring and analysis, the platform streamlines workflows, simplifies trial oversight, and accelerates decision-making processes, enabling sponsors and research teams to focus on trial outcomes.